Enantioselective synthesis of P-chiral tertiary phosphine oxides with an ethynyl group via Cu(i)-catalyzed azide–alkyne cycloaddition†
Abstract
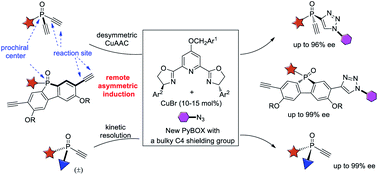
We report the highly enantioselective synthesis of P-chiral tertiary phosphine oxides featuring an ethynyl group via Cu(I)-catalyzed azide–alkyne cycloaddition. Newly developed chiral pyridinebisoxazolines (PYBOX) bearing a bulky C4 shielding group play an important role in achieving excellent enantioselectivity while suppressing side bis-triazoles formation in desymmetrizing prochiral diethynylphosphine oxides. Notably, by tuning the size of the C4 shielding group, it is possible to achieve excellent remote enantiofacial control in desymmetrizing phosphole oxide-diynes with the prochiral P-center farther from the ethynyl group by four covalent bonds. Time-dependent enantioselectivity is observed for these desymmetric CuAAC reactions, suggesting a synergic combination of a desymmetrization and a kinetic resolution, and our ligands prove to be better than unmodified PYBOX in both steps. This finding contributes to a highly enantioselective kinetic resolution of racemic ethynylphosphine oxides. The resulting chiral ethynylphosphine oxides are versatile P-chiral synthons, which can undergo a number of diversifying reactions to enrich structural diversity.
- This article is part of the themed collection: 2019 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...