Rational synthesis of interpenetrated 3D covalent organic frameworks for asymmetric photocatalysis†
Abstract
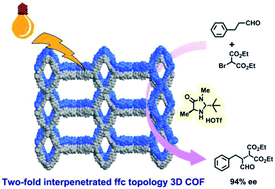
Covalent organic frameworks (COFs) show great promise as heterogeneous photocatalysts, but they have not yet been explored for asymmetric photocatalysis, which is important for the sustainable production of pharmaceuticals and fine chemicals. We report here a pair of twofold interpenetrated 3D COFs adopting a rare (3,4)-connected ffc topology for photocatalytic asymmetric reactions by imine condensation of rectangular and trigonal building blocks. Both COFs containing a photoredox triphenylamine moiety are efficient photocatalysts for the cross-dehydrogenative coupling reactions and asymmetric α-alkylation of aldehydes integrated with a chiral imidazolidinone catalyst. Under visible-light irradiation, the targeted chiral products are produced in satisfactory yields with up to 94% enantiomeric excess, which are comparable to those of reported reactions using molecular metal complexes or organic dyes as photosensitizers. Whereas the COFs became amorphous after catalysis, they can be recrystallized through solvent-assisted linker exchange and reused without performance loss. This is the first report utilizing COFs as photocatalysts to promote enantioselective photochemical reactions.
- This article is part of the themed collections: Most popular 2019-2020 materials and energy chemistry articles and In celebration of Chinese New Year


 Please wait while we load your content...
Please wait while we load your content...