A possible unaccounted source of atmospheric sulfate formation: amine-promoted hydrolysis and non-radical oxidation of sulfur dioxide†
Abstract
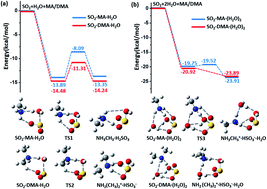
Numerous field and laboratory studies have shown that amines, especially dimethylamine (DMA), are crucial to atmospheric particulate nucleation. However, the molecular mechanism by which amines lead to atmospheric particulate formation is still not fully understood. Herein, we show that DMA molecules can also promote the conversion of atmospheric SO2 to sulfate. Based on ab initio simulations, we find that in the presence of DMA, the originally endothermic and kinetically unfavourable hydrolysis reaction between gaseous SO2 and water vapour can become both exothermic and kinetically favourable. The resulting product, bisulfite NH2(CH3)2+·HSO3−, can be readily oxidized by ozone under ambient conditions. Kinetic analysis suggests that the hydrolysis rate of SO2 and DMA with water vapour becomes highly competitive with and comparable to the rate of the reaction between SO2 and OH·, especially under the conditions of heavily polluted air and high humidity. We also find that the oxidants NO2 and N2O5 (whose role in sulfate formation is still under debate) appear to play a much less significant role than ozone in the aqueous oxidation reaction of SO2. The newly identified oxidation mechanism of SO2 promoted by both DMA and O3 provides another important new source of sulfate formation in the atmosphere.


 Please wait while we load your content...
Please wait while we load your content...