A novel photoredox-active group for the generation of fluorinated radicals from difluorostyrenes†
Abstract
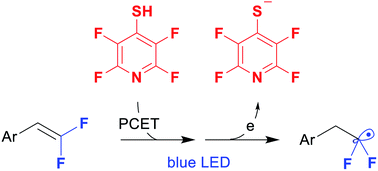
A 4-tetrafluoropyridinylthio group was suggested as a new photoredox-active moiety. The group can be directly installed on difluorostyrenes in a single step by the thiolene click reaction. It proceeds upon visible light catalysis with 9-phenylacridine providing various difluorinated sulfides as radical precursors. Single electron reduction of the C–S bond with the formation of fluoroalkyl radicals is enabled by the electron-poor azine ring. The intermediate difluorinated sulfides were involved in a series of photoredox reactions with silyl enol ethers, alkenes, nitrones and an alkenyl trifluoroborate.


 Please wait while we load your content...
Please wait while we load your content...