From 1,2-difunctionalisation to cyanide-transfer cascades – Pd-catalysed cyanosulfenylation of internal (oligo)alkynes†
Abstract
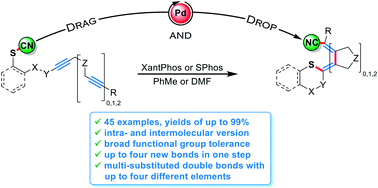
Internal alkynes substituted by aliphatic or aromatic moieties or by heteroatoms were converted into sulphur-substituted acrylonitrile derivatives. Key is the use of Pd catalysis, which allows the addition of aromatic and aliphatic thiocyanates in an intra- and intermolecular manner. Substrates with several alkyne units underwent further carbopalladation steps after the initial thiopalladation step, thus generating in a cascade-like fashion an oligoene unit with sulphur at one terminus and the cyano group at the other.


 Please wait while we load your content...
Please wait while we load your content...