Catalytic asymmetric hydrogenation of (Z)-α-dehydroamido boronate esters: direct route to alkyl-substituted α-amidoboronic esters†
Abstract
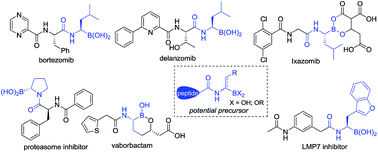
The direct catalytic asymmetric hydrogenation of (Z)-α-dehydroamino boronate esters was realized. Using this approach, a class of therapeutically relevant alkyl-substituted α-amidoboronic esters was easily synthesized in high yields with generally excellent enantioselectivities (up to 99% yield and 99% ee). The utility of the products has been demonstrated by transformation to their corresponding boronic acid derivatives by a Pd-catalyzed borylation reaction and an efficient synthesis of a potential intermediate of bortezomib. The clean, atom-economic and environment friendly nature of this catalytic asymmetric hydrogenation process would make this approach a new alternative for the production of alkyl-substituted α-amidoboronic esters of great potential in the area of organic synthesis and medicinal chemistry.


 Please wait while we load your content...
Please wait while we load your content...