Redox, transmetalation, and stacking properties of tetrathiafulvalene-2,3,6,7-tetrathiolate bridged tin, nickel, and palladium compounds†
Abstract
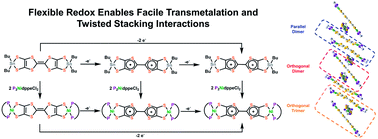
Here we report that capping the molecule TTFtt (TTFtt = tetrathiafulvalene-2,3,6,7-tetrathiolate) with dialkyl tin groups enables the isolation of a stable series of redox congeners and facile transmetalation to Ni and Pd. TTFtt has been proposed as an attractive building block for molecular materials for two decades as it combines the redox chemistry of TTF and dithiolene units. TTFttH4, however, is inherently unstable and the incorporation of TTFtt units into complexes or materials typically proceeds through the in situ generation of the tetraanion TTFtt4−. Capping of TTFtt4− with Bu2Sn2+ units dramatically improves the stability of the TTFtt moiety and furthermore enables the isolation of a redox series where the TTF core carries the formal charges of 0, +1, and +2. All of these redox congeners show efficient and clean transmetalation to Ni and Pd resulting in an analogous series of bimetallic complexes capped by 1,2-bis(diphenylphosphino)ethane (dppe) ligands. Furthermore, by using the same transmetalation method, we synthesized analogous palladium complexes capped by 1,1′-bis(diphenylphosphino)ferrocene (dppf) which had been previously reported. All of these species have been thoroughly characterized through a systematic survey of chemical and electronic properties by techniques including cyclic voltammetry (CV), ultraviolet-visible-near infrared spectroscopy (UV-vis-NIR), electron paramagnetic resonance spectroscopy (EPR), nuclear magnetic resonance spectroscopy (NMR) and X-ray diffraction (XRD). These detailed synthetic and spectroscopic studies highlight important differences between the transmetalation strategy presented here and previously reported synthetic methods for the installation of TTFtt. In addition, the utility of this stabilization strategy can be illustrated by the observation of unusual TTF radical–radical packing in the solid state and dimerization in the solution state. Theoretical calculations based on variational 2-electron reduced density matrix methods have been used to investigate these unusual interactions and illustrate fundamentally different levels of covalency and overlap depending on the orientations of the TTF cores. Taken together, this work demonstrates that tin-capped TTFtt units are ideal reagents for the installation of redox-tunable TTFtt ligands enabling the generation of entirely new geometric and electronic structures.
- This article is part of the themed collection: Tin Collection – a celebration of our 10th anniversary


 Please wait while we load your content...
Please wait while we load your content...