Bidirectional enantioselective synthesis of bis-benzofuran atropisomeric oligoarenes featuring two distal C–C stereogenic axes†
Abstract
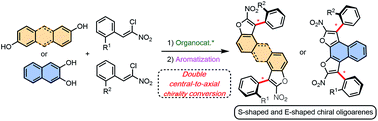
We report the bidirectional enantioselective synthesis of bis-benzofuran atropisomeric oligoarenes featuring two distal C–C stereogenic axes obtained by a two-fold central-to-axial chirality conversion upon oxidative aromatization. The key enantioenriched centrally chiral bis-dihydrobenzofuran precursors were synthesized via a bidirectional diastereo- and enantio-selective organocatalyzed domino reaction between simple achiral and easily accessible dihydroxylated aromatics and chloronitroalkenes. Moreover, the stereodivergent nature of the methodology was established by synthesizing both diastereomers of a non-symmetrically functionalized bis-axially chiral oligoarene.
- This article is part of the themed collections: In celebration of Chinese New Year and Most popular 2019-2020 supramolecular chemistry articles


 Please wait while we load your content...
Please wait while we load your content...