Mechanistic insights into copper-catalyzed aerobic oxidative coupling of N–N bonds†
Abstract
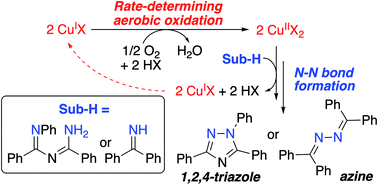
Catalytic N–N coupling is a valuable transformation for chemical synthesis and energy conversion. Here, mechanistic studies are presented for two related copper-catalyzed oxidative aerobic N–N coupling reactions, one involving the synthesis of a pharmaceutically relevant triazole and the other relevant to the oxidative conversion of ammonia to hydrazine. Analysis of catalytic and stoichiometric N–N coupling reactions support an “oxidase”-type catalytic mechanism with two redox half-reactions: (1) aerobic oxidation of a CuI catalyst and (2) CuII-promoted N–N coupling. Both reactions feature turnover-limiting oxidation of CuI by O2, and this step is inhibited by the N–H substrate(s). The results highlight the unexpected facility of the N–N coupling step and establish a foundation for development of improved catalysts for these transformations.


 Please wait while we load your content...
Please wait while we load your content...