2-Amino-3′-dialkylaminobiphenyl-based fluorescent intracellular probes for nitric oxide surrogate N2O3†
Abstract
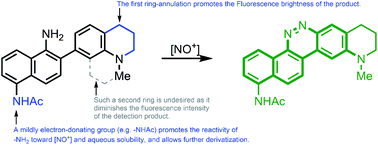
Fluorescent probes for nitric oxide (NO), or more frequently for its oxidized surrogate dinitrogen trioxide (N2O3), have enabled scientists to study the contributions of this signaling molecule to many physiological processes. Seeking to improve upon limitations of other probes, we have developed a family of fluorescent probes based on a 2-amino-3′-dialkylaminobiphenyl core. This core condenses with N2O3 to form benzo[c]cinnoline structures, incorporating the analyte into the newly formed fluorophore, which results in product fluorescence with virtually no background contribution from the initial probe. We varied the substituents in the core in order to optimize both the reactivity of the probes with N2O3 and their cinnoline products' fluorescence wavelengths and brightness. The top candidates were then applied to cultured cells to verify that they could respond to NO within cellular milieus, and the top performer, NO530, was compared with a “gold standard” commercial probe, DAF-FM, in a macrophage-derived cell line, RAW 264.7, stimulated to produce NO. NO530 demonstrated similar or better sensitivity and higher selectivity for NO than DAF, making it an attractive potential alternative for NO tracking in various applications.


 Please wait while we load your content...
Please wait while we load your content...