Zn-mediated decarboxylative carbagermatranation of aliphatic N-hydroxyphthalimide esters: evidence for an alkylzinc intermediate†
Abstract
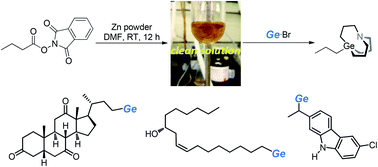
Alkyl nucleophiles synthesized by decarboxylation of the corresponding N-hydroxyphthalimide esters (NHP esters) would inherit the complex structure of natural carboxylic acids and result in useful cross-coupling fragments. Herein, we report the synthesis of alkyl carbagermatranes via Zn-mediated decarboxylation of NHP esters without Ni catalysis or photocatalysis. Mechanistic studies indicate that an alkyl zinc intermediate was involved; however, the generation of alkyl zinc will be inhibited in the presence of Ni. Hence, this study provides valuable resolution to the perplexing problem about whether organozinc was involved in recently emerging catalytic systems of NHP ester–Zn. Meanwhile, alkyl zinc reagents from NHP esters are compatible with aryl/alkyl bromides and iodides; therefore the scope of carbagermatranation in this work precedes that of in situ-generated organozinc from alkyl halides.


 Please wait while we load your content...
Please wait while we load your content...