Abstract
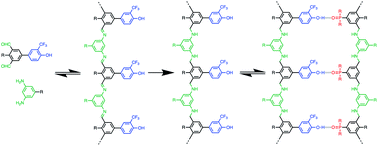
A new family of recognition-encoded oligomers that form stable duplexes in chloroform have been prepared. Monomer building blocks composed of dialdehydes functionalised with either a trifluoromethylphenol or phosphine oxide H-bond recognition unit were prepared. The dialdehydes were coupled with diamines by imine formation and then reduction to give homo-oligomers between one and three recognition units in length. Duplex formation was characterised by 19F and 1H NMR titration experiments in toluene and in chloroform. For duplexes formed between length complementary H-bond donor and acceptor homo-oligomers, an order of magnitude increase in stability was observed for every base-pair added to the duplex in chloroform. The effective molarity for the intramolecular H-bonds responsible for zipping up the duplex is 30 mM, which results in the fully assembled duplex in all cases. The uniform increase in duplex stability with oligomer length suggests that the backbone structure and geometry is likely to be compatible with the formation of extended duplexes in longer oligomers.


 Please wait while we load your content...
Please wait while we load your content...