Borane-induced ring closure reaction of oligomethylene-linked bis-allenes†
Abstract
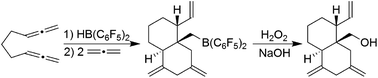
The trimethylene-linked bis-allene 3a reacts with Piers' borane [HB(C6F5)2] by a hydroboration/allylboration sequence to generate the cyclization product 5a. Its pyridine adduct was isolated and characterized by X-ray diffraction. Compound 5a undergoes a typical frustrated Lewis pair 1,2-P/B alkene addition reaction with PPh3 to give the heterobicyclic bridged olefinic zwitterionic product 9a. The tetramethylene-linked bis-allene 3b and its phenylene annulated analogue 3c react with HB(C6F5)2 to give the analogous seven-membered ring products 5b,c under mild conditions. The cyclization product 5a undergoes a series of sequential allylboration reactions with two equivalents of allene followed by ring-closure to give the four-component coupling product 12a. It undergoes FLP addition to an exo-methylene group upon treatment with PPh3. Compound 12a is oxidatively converted to the boron-free alcohol.


 Please wait while we load your content...
Please wait while we load your content...