Formyl-selective deuteration of aldehydes with D2O via synergistic organic and photoredox catalysis†
Abstract
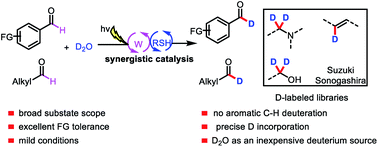
Formyl-selective deuteration of aldehydes is of high interest for labeling purposes and for optimizing properties of drug candidates. Herein, we report a mild general method for formyl-selective deuterium labeling of aldehydes with D2O, an inexpensive deuterium source, via a synergistic combination of light-driven, polyoxometalate-facilitated hydrogen atom transfer and thiol catalysis. This highly efficient, scalable reaction showed excellent deuterium incorporation, a broad substrate scope, and excellent functional group tolerance and selectivity and is therefore a practical method for late-stage modification of synthetic intermediates in medicinal chemistry and for generating libraries of deuterated compounds.
- This article is part of the themed collections: Celebrating 100 Years of Chemistry at Nankai University, Most popular 2019-2020 organic chemistry articles and In celebration of Chinese New Year


 Please wait while we load your content...
Please wait while we load your content...