Harnessing interrupted Fischer in continuous flow: sustainable synthesis of (spiro)indolenine and (spiro)indoline privileged scaffolds†
Abstract
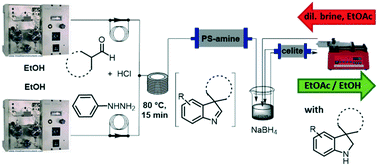
A greener and sustainable flow chemistry protocol for the synthesis of 3,3-disubstituted indolenines through interrupted Fischer indolisation reaction is described. First, two model aldehydes were reacted with phenylhydrazine in order to explore the reaction feasibility in a ‘greener’ fashion in batch mode. The best outcomes were then used as the starting point for the implementation of the reaction in continuous flow. A thorough exploration of key parameters allowed the identification of the most efficient reagent mixing mode, and the optimum temperature and residence time. The newly developed method allowed straightforward reaction channelling towards the formation of the indolenines, thus reducing the competitive formation of side products. We further broadened the scope of the conceived methodology by exploring the possibility of a heterogeneous in-line reduction of the indolenines to their indoline counterparts. This rapid approach nicely complements known batch chemistry and could facilitate synthesis and scale up of 3,3-disubstituted indolenines and indolines, offering a coupling point for additional and subsequent flow reactions for multistep syntheses for further derivatization.


 Please wait while we load your content...
Please wait while we load your content...