Synthesis of 3-trifluoromethylated 1,3-butadienes via a Pd(0)-catalyzed fluorinated Heck reaction†
Abstract
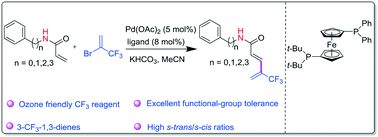
1,3-Butadienes play a key role in modern synthetic chemistry and biochemistry because they are well-known precursors for the synthesis of retinoids. A new and efficient synthesis of 3-trifluoromethylated 1,3-butadienes via a Pd(0)-catalyzed fluorinated Heck reaction is described. Without an additive, the reaction of 2-bromo-3,3,3-trifluoropropene, an abundant and ozone friendly CF3 building block, with acrylamides afforded 3-trifluoromethylated 1,3-butadienes in good to excellent yields with high regioselectivity and s-trans/s-cis ratios under mild conditions. Inspired by the concept of space–time yield for continuous reactions, the average space–time yield calculated shows that all the reactions far exceed 1 g gcat−1 h−1, which is high enough for further scale-up, suggesting that small amounts of catalyst and a very small reactor are required.


 Please wait while we load your content...
Please wait while we load your content...