Minimization of Pt-electrocatalyst deactivation in CO2 reduction using a polymer electrolyte cell†
Abstract
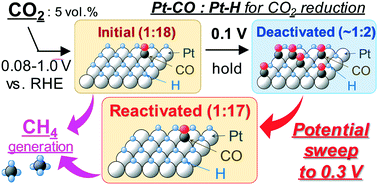
Diluted CO2 feeding was recently reported to efficiently generate CH4 at the theoretical Pt electrode potential, however, the reaction was easily deactivated. To solve this problem, we investigated the reaction/deactivation mechanism to produce CH4 from CO2 electroreduction. Using a polymer electrolyte single cell containing a Pt/C catalyst, CO2 was reduced to CH4 without overpotential by simply controlling the CO2 feed concentration. The CH4 synthesis proceeded if the Pt–CO/Pt–H ratio formed on the Pt-catalyst surface was 1 : 11 or higher. The deactivation of the CH4 generating reaction also depends on the Pt–CO/Pt–H ratio (the ratio does not satisfy 1 : 11 or higher). The optimum Pt–CO/Pt–H ratio to produce CH4 was 1 : 18. Furthermore, we achieved 86% recovery of CH4 activity by sweeping the deactivated Pt surface on the cathode up to 0.3 V where the CO2/Pt–CO redox reaction occurred simultaneously. As a result, an efficient less energy-intensive reactivation reaction that we defined as a poisoning-elimination method was established. Overall, this work demonstrated that the application of a polymer electrolyte cell together with a low concentration of CO2 is effective to minimize Pt-electrocatalyst deactivation.


 Please wait while we load your content...
Please wait while we load your content...