Hydrogenolysis of lignin-derived aryl ethers to monomers over a MOF-derived Ni/N–C catalyst†
Abstract
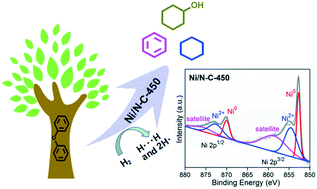
A highly efficient Ni/N–C catalyst was synthesized by facile pyrolysis of a Ni-containing metal–organic framework (Ni-MOF), and its catalytic hydrogenolysis performance towards C–O bonds in lignin was evaluated in detail using diphenyl ether (DPE) as a model compound. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) analyses show that the flower-like nanosheets of the Ni-MOF shrink, forming a loose and ordered spherical structure during pyrolysis. Under the optimal conditions, DPE was completely converted and the selectivity to monomers (benzene, cyclohexanol and cyclohexane) reached 99.1%. During the catalytic hydrogenolysis conversion (CHC) of DPE, the direct cleavage of the Caromatic–O bond affording benzene and phenol is the major reaction pathway, and a low H2 pressure is crucial to increase the monomer selectivity. Furthermore, Ni/N–C-450 shows high hydrogenolysis activity for other lignin-derived aryl ethers, such as benzyl phenyl ether, dibenzyl ether, dinaphthalene ether, benzyl 2-naphthyl ether and 3-methoxyphenol.


 Please wait while we load your content...
Please wait while we load your content...