Theoretical study on enzyme synthesis of cephalexin in a parallel-flow microreactor combined with electrically driven ATPS microextraction
Abstract
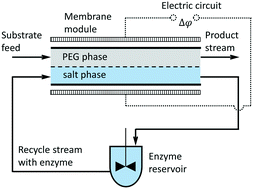
Cephalexin is an important β-lactam antibiotic that is enzymatically synthetized from a nucleophile (7-aminodeacetoxycephalosporanic acid – 7-ADCA) and an acyl donor (phenylglycine methyl ester – PGME). The process is catalyzed by penicillin acylase. Cephalexin is thermodynamically unstable and is typically produced in a kinetic regime. Based on our previous experimental findings and additional batch experiments intended for the estimation of kinetic constants of cephalexin synthesis, we developed a mathematical model of a microfluidic device with two aqueous phases (ATPS) for the simultaneous cephalexin production and its separation from a reaction mixture. This device operates with free enzyme dissolved in one phase and the reactants introduced in the other phase. Because of small characteristic dimensions, the reactants are intensively transported through the interface to the enzyme phase where they are converted to cephalexin. The product then easily returns into the original phase due to a high value of the partition coefficient. The transport can be enhanced by an imposed electric field as the reaction compounds are charged. We studied the effects of four well-controllable parameters on the cephalexin yield: (i) the residence time of the phase introducing the reactants, (ii) the residence time of the phase containing the enzyme, (iii) the applied voltage difference across the interface, (iv) the characteristic dimension of microfluidic chambers. The mathematical model predicts that a cephalexin yield higher than 70% can be achieved in counter-current parallel flow arrangement, which is a result comparable with those obtained in batch experiments. The applied electric field can increase the cephalexin yield by no more than several percent because of the same polarity of 7-ADCA and cephalexin charge numbers. If compared to classical batch reactors, the suggested microreactor–microseparator brings the following benefits: (i) continuous cephalexin synthesis, (ii) effective and continuous separation of cephalexin due to proper partitioning of these species in the used ATPS, (iii) the use of free and highly active enzyme with efficient recyclation. Moreover, the productivity of the suggested microreactor is solely determined by the interfacial area that can be easily provided by thin separating membranes, i.e. no technically demanding numbering up solution is necessary.

 Please wait while we load your content...
Please wait while we load your content...