A chemo-enzymatic tandem reaction in a mixture of deep eutectic solvent and water in continuous flow†
Abstract
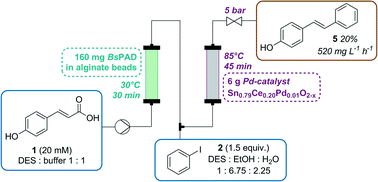
The combination of metal- and biocatalysis is a challenging but forward-looking topic in synthetic chemistry. The unique selectivity of enzymes paired with the broad range of applications of chemical catalysts enables an undreamed-of number of novel processes. Herein, we describe the application of immobilized phenolic acid decarboxylase (PAD) for the decarboxylation of para-coumaric acid and subsequent Pd-catalyzed Heck cross-coupling with an aryl halide in a fully integrated two-step continuous flow process to synthesize (E)-4-hydroxy-stilbene. The application of a choline chloride-based deep eutectic solvent (DES) proved to be crucial to overcome the problem of solvent compatibility and enabled an increase in substrate concentration (from 5 mM in buffer to 20 mM in DES) as well as a process with a homogeneous starting solution. The two-step process was successfully operated for more than 16 h in continuous flow and full conversion was achieved. The results underline the usefulness of DES to overcome compatibility problems in tandem-catalytic processes. The system benefits from its simplicity due to increased substrate solubility, the possibility to conduct both reactions at their optimal temperatures and the elimination of isolating the reaction intermediate, which is prone to polymerization.


 Please wait while we load your content...
Please wait while we load your content...