Experimental and computational investigations of C–H activation of cyclohexane by ozone in liquid CO2†
Abstract
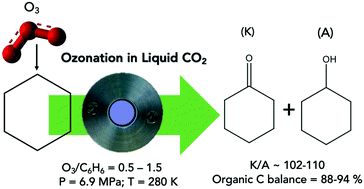
Facile cyclohexane oxidation by ozone in liquid CO2 was demonstrated in a Parr reactor equipped with in situ infrared probe. The dominant presence of CO2 in the vapor phase allows operation below the lower flammability limit. At 280 K, 6.9 MPa and a molar feed O3/cyclohexane ratio ≃0.5, the cyclohexane conversion was ∼12% during a 1 h batch run with cyclohexanone (K) + cyclohexanol (A) yield and K/A being ∼11% and ∼100, respectively. Small but measurable amounts of hydrogen peroxide were also formed. The absence of any detectable water in the product and the close match of cyclohexane conversion and product yield rule out significant cyclohexane combustion. The observed products are consistent with reaction pathways predicted by density functional theory (DFT) computations and involve the formation of a hydrotrioxide (HO3C6H11) intermediate. The DFT-computed energetics of the initial hydrogen-atom transfer reaction between O3 and cyclohexane to give the hydrotrioxide were validated by comparison with multi-reference complete active space self-consistent field (CASSCF) computations, which included n-electron valence state perturbation theory (NEVPT2) for dynamic correlation. Even though DFT cannot properly treat the multireference character of O3, the agreement between these methods was reasonable. Preliminary studies of isooctane ozonation demonstrate that ozone is capable of activating all carbons (with a preference for the primary carbon) producing a variety of oxygenated products (alcohols, ketones and acid) with negligible formation of combustion products. These results clearly demonstrate the ability of ozone in selectively oxidizing C–H bonds in alkanes.


 Please wait while we load your content...
Please wait while we load your content...