Metal- and solvent-free synthesis of aminoalcohols under continuous flow conditions†
Abstract
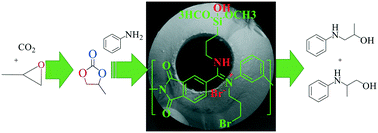
The use of multifunctional organocatalysts and continuous flow platforms are commonplace in modern chemical transformation. Herein, we describe a method for immobilization of trifunctional organocatalysts on porous composite hollow fibers and demonstrate their application as heterogeneous catalysts and continuous-flow microfluidic reactors for chemical transformation. Polyamide-imide hollow fibers (PAIHFs) are functionalized with aminosilanes and a bromine source to immobilize covalent hydrogen-bond donor groups (–OH and –NH) and nucleophilic [Br−] species on the fiber surface and provide trifunctional acid–base–nucleophilic organocatalysts and microfluidic reactors. The cooperative effects of the Br/APS/PAIHF trifunctional organocatalysts are elucidated in the CO2 cycloaddition and hydroxyalkylation of aniline in batch and continuous flow synthesis. Our results indicated that the synergistic cooperative effects of trifunctional organocatalysts on PAIHFs lead to a maximum 1-(phenylamino)propan-2-ol selectivity of 97.1% at 61% aniline conversion and 0.02 cm3 min−1 flow rate. While knowledge of the acid–base–nucleophilic trifunctional cooperativity is still limited, these findings demonstrate useful structure–property trends that can be used to design more efficient organocatalysts for sustainable chemical transformation.


 Please wait while we load your content...
Please wait while we load your content...