Kinetic modeling and mechanistic investigations of transesterification of propylene carbonate with methanol over an Fe–Mn double metal cyanide catalyst†
Abstract
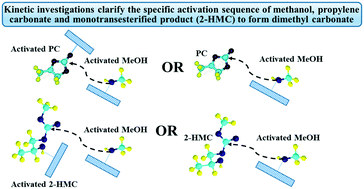
Kinetic modeling of transesterification of propylene carbonate with methanol using an Fe–Mn double metal cyanide catalyst has been investigated based on experimental data obtained under kinetically controlled conditions in a batch slurry reactor in the 140–200 °C range. A simple two-step power law model was found to represent the experimental data well. In addition, a detailed kinetic model based on a molecular level description of the reaction mechanism is also evaluated to provide better insight into the reaction mechanism. It is found that a kinetic model based on the following mechanistic steps provides the best description of the experimental data: (a) activation of methanol by the catalyst to form a methoxy intermediate, (b) activation of propylene carbonate by interaction with the methoxy intermediate to form a second intermediate, (c) reaction of the second intermediate with methoxy species to form a third intermediate which decomposes into the final products dimethyl carbonate and propylene glycol with regeneration of the catalyst precursor. The kinetic model along with mechanistic insights from the present study provides rational guidance for catalyst redesign and process optimization.


 Please wait while we load your content...
Please wait while we load your content...