The role of hydrogen coverage and location in 1,3-butadiene hydrogenation over Pt/SiO2†
Abstract
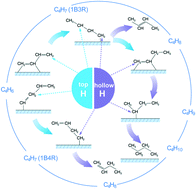
Hydrogenation of 1,3-butadiene over supported Pt particles has long been known as a structure sensitive reaction, yet the nature of this phenomenon has not been well understood. We have previously addressed the reaction pathway for 1,3-butadiene hydrogenation over ∼22 nm Pt particles. In this study, the behaviors of SiO2 supported ∼2.9 nm Pt particles towards 1,3-butadiene hydrogenation have been investigated with the aim of providing a fundamental explanation for the structure sensitivity of this reaction. Interestingly, it was found that the product distribution for the present catalyst towards 1,3-butadiene hydrogenation was sensitive to temperature. However, the evolution of 1,3-butadiene hydrogenation over the catalyst with temperature was not captured by in situ diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy. The detected carbonaceous species on the Pt surface are more likely to be spectators rather than reaction intermediates for the formation of butenes and n-butane. To understand the behaviors of the catalyst, density functional theory (DFT) calculations were employed to explore the pathways for the formation of the products with low activation barriers. The results show that the coverage and the location of the hydrogen atom are the two key factors for the interpretation of the behavior of Pt particles towards 1,3-butadiene hydrogenation.


 Please wait while we load your content...
Please wait while we load your content...