Precise synthesis of amphiphilic diblock copolymers consisting of various ionic liquid-type segments and their influence on physical gelation behavior in water†
Abstract
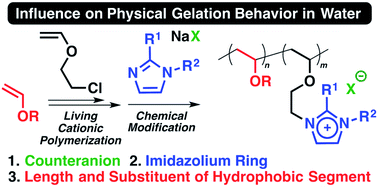
Appropriately designed amphiphilic diblock vinyl ether (VE) copolymers consisting of an ionic liquid-type segment and a hydrophobic segment were demonstrated to undergo physical gelation in water at extremely low concentrations. The precursor diblock copolymers were synthesized by the living cationic polymerization of 2-chloroethyl VE with a hydrophobic VE through an appropriately designed initiating system such as optimized temperature and catalyst. A relatively high temperature such as 20 °C was important for controlled polymerization of octadecyl VE. Ionic liquid moieties with imidazolium salt structures were subsequently introduced into the side chains of the diblock copolymers via chemical modifications of the 2-chloroethyl groups. The physical gelation behavior of the obtained diblock copolymers was examined in water, with a particular focus on the influence of the hydrophobic VEs, the hydrophilicity of the counteranions and the substituents on the ionic liquid-type segments, and the length of each segment. Based on this systematic investigation, physical gelation at concentrations as low as 0.2 wt% was achieved with diblock copolymers with a suitable balance of these factors.


 Please wait while we load your content...
Please wait while we load your content...