Applications of tert-butanesulfinamide in the synthesis of N-heterocycles via sulfinimines
Abstract
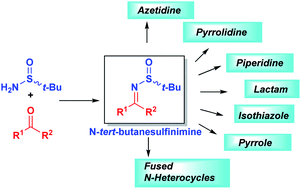
Chiral sulfinamides are among the best known chiral auxiliaries in the stereoselective synthesis of amines and their derivatives. The most extensively used enantiopure tert-butanesulfinamide emerged as the gold standard among many others over the last two decades. The present review attempts to provide an overview of tert-butanesulfinamide mediated asymmetric N-heterocycle synthesis via sulfinimines and covers literature from 2010–2020. This methodology offers general access to structurally diverse piperidines, pyrrolidines, azetidines, and their fused derivatives that represent the structural motif of many natural products and therapeutically applicable compounds.
- This article is part of the themed collection: 2020 Reviews in RSC Advances


 Please wait while we load your content...
Please wait while we load your content...