Co-assembly of charge complementary peptides and their applications as organic dye/heavy metal ion (Pb2+, Hg2+) absorbents and arsenic(iii/v) detectors†
Abstract
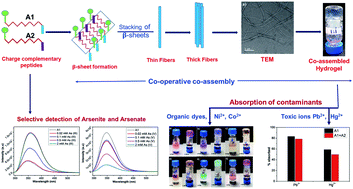
Learning from nature, molecular self-assembly has been used extensively to generate interesting materials using a bottom up approach. The enthusiasm in this field of research stems from the unique properties of these materials and their diverse applications. The field has not been limited to studying assembly of similar types of molecules but extended to multi component systems via the co-assembly phenomenon. We have designed two charge complementary peptides to study their co-assembly in mechanistic detail in the present work. The cooperative self-assembly is mainly driven by electrostatic interaction that is aided by aromatic interactions, hydrogen bonding interactions and hydrophobic interactions. The hydrogels obtained have been employed in waste water remediation. Both the self-assembled and co-assembled hydrogels are capable of removal of different kinds of organic dyes (cationic, anionic and neutral) and toxic metal ions (Ni2+, Co2+, Pb2+ and Hg2+) individually and as a mixture from water with high efficiency. Additionally, the peptides developed in this study can act as ion sensors and detect arsenic in its most toxic (III/V) oxidation states. Molecular understanding of the assembly process is of fundamental importance in the rational design of such simple, robust yet economically viable materials with versatile and novel applications.


 Please wait while we load your content...
Please wait while we load your content...