Polyaniline and sodium alginate nanocomposite: a pH-responsive adsorbent for the removal of organic dyes from water†
Abstract
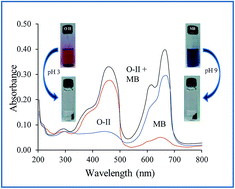
pH-responsive adsorbents are promising tools in water remediation as they possess selective adsorption towards cationic and anionic dyes, which can be controlled by varying the pH of the medium. In this study, a pH-responsive nanocomposite of polyaniline–sodium alginate (PANI/SA) was synthesized. The composite was found to be an efficient adsorbent for the removal of both cationic and anionic dyes from water at different pH values. The extent of adsorption was evaluated as a function of initial dye concentration, solution pH, temperature, contact time, dose of adsorbent and coexisting ions. The detailed investigation of kinetics and adsorption isotherm showed that the dyes adsorbed in accordance with pseudo-second order kinetic and Langmuir adsorption isotherms. The maximum adsorption capacities of the nanocomposite for Methylene blue (MB), Rhodamine B (RB), Orange-II (O-II), and Methyl orange (MO) dyes were found to be 555.5 mg g−1, 434.78 mg g−1, 476.19 mg g−1, and 416.66 mg g−1, respectively, which is higher compared to other reported adsorbents. The feasibility of the adsorption process was ascertained from thermodynamic parameters.


 Please wait while we load your content...
Please wait while we load your content...