Synergistic effect of Ni–Ag–rutile TiO2 ternary nanocomposite for efficient visible-light-driven photocatalytic activity†
Abstract
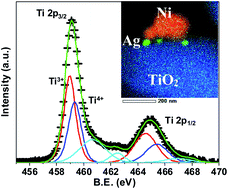
P25 comprising of mixed anatase and rutile phases is known to be highly photocatalytically active compared to the individual phases. Using a facile wet chemical method, we demonstrate a ternary nanocomposite consisting of Ni and Ag nanoparticles, decorated on the surface of XTiO2 (X: P25, rutile (R)) as an efficient visible-light-driven photocatalyst. Contrary to the current perspective, RTiO2-based Ni–Ag–RTiO2 shows the highest activity with the H2 evolution rate of ∼86 μmol g−1 W−1 h−1@535 nm. Together with quantitative assessment of active Ni, Ag and XTiO2 in these ternary systems using high energy synchrotron X-ray diffraction, transmission electron microscopy coupled energy dispersive spectroscopy mapping evidences the metal to semiconductor contact via Ag. The robust photocatalytic activity is attributed to the improved visible light absorption, as noted by the observed band edge of ∼2.67 eV corroborating well with the occurrence of Ti3+ in Ti 2p XPS. The effective charge separation due to intimate contact between Ni and RTiO2 via Ag is further evidenced by the plasmon loss peak in Ag 3d XPS. Moreover, density functional theory calculations revealed enhanced adsorption of H2 on Ti8O16 clusters when both Ag and Ni are simultaneously present, owing to the hybridization of the metal atoms with d orbitals of Ti and p orbitals of O leading to enhanced bonding characteristics, as substantiated by the density of states. Additionally, the variation in the electronegativity in Bader charge analysis indicates the possibility of hydrogen evolution at the Ni sites, in agreement with the experimental observations.


 Please wait while we load your content...
Please wait while we load your content...