A study on the selective catalytic reduction of NOx by ammonia on sulphated iron-based catalysts
Abstract
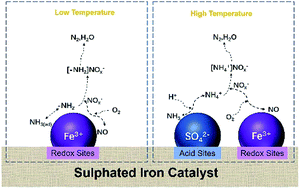
A series of sulphated iron-based catalysts was prepared via an impregnation method by changing the loading content of Fe3+ and SO42− on ZrO2, and their performance in the selective catalytic reduction (SCR) of NOx by ammonia was investigated. The NOx conversion exhibited large differences among the sulphated iron-based catalysts. To explore the synergistic mechanism of iron and sulphates, XRD, BET, H2-TPR, XPS, TPD and in situ DRIFTS were used to characterize the catalysts, and it was found that among all the catalysts, the NOx conversion by Fe2SZr was greater than 90% at 350–450 °C. The results indicated that the interaction between Fe3+ and SO42− can have an effect on the redox ability, acid sites, and adsorption of NOx and NH3. With an increase in the content of Fe3+, the redox activity of the catalyst and the adsorption of ammonia improved at medium and low temperatures. However, at higher temperatures, an increase in Fe3+ led to a decrease in the conversion of NOx due to the enhanced oxidation of NH3. At medium and low temperatures, an increase in the content of SO42− decreased the concentration of Fe3+ on the surface of the catalyst and inhibited the adsorption of NOx and NH3. The addition of SO42− reduced the redox activity of the catalyst and inhibited the oxidation reaction of NH3, which follows the Eley–Rideal mechanism at high temperatures, further enhancing the SCR activity of the FexSyZr catalyst.
- This article is part of the themed collection: How does it work? – a collection on mechanistic studies


 Please wait while we load your content...
Please wait while we load your content...