Recent advances in the pharmacological diversification of quinazoline/quinazolinone hybrids
Abstract
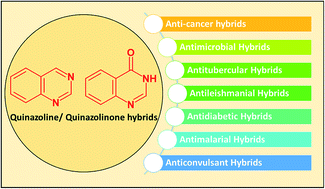
Due to the pharmacological activities of quinazoline and quinazolinone scaffolds, it has aroused great interest in medicinal chemists for the development of new drugs or drug candidates. The pharmacological activities of quinazoline and its related scaffolds include anti-cancer, anti-microbial, anti-convulsant, and antihyperlipidaemia. Recently, molecular hybridization technology is used for the development of hybrid analogues with improved potency by combining two or more pharmacophores of bioactive scaffolds. The molecular hybridization of various biologically active pharmacophores with quinazoline derivatives resulted in lead compounds with multi-faceted biological activity wherein specific as well as multiple targets were involved. The present review summarizes the advances in lead compounds of quinazoline hybrids and their related heterocycles in medicinal chemistry. Moreover, the review also helps to intensify the drug development process by providing an understanding of the potential role of these hybridized pharmacophoric features in exhibiting various pharmacological activities.
- This article is part of the themed collection: 2020 Reviews in RSC Advances


 Please wait while we load your content...
Please wait while we load your content...