Chemical grafting of cholesterol on monomer and PDMMLA polymers, a step towards the development of new polymers for biomedical applications
Abstract
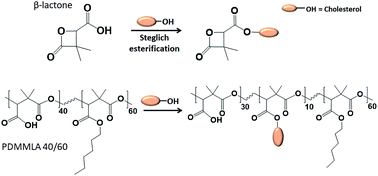
Racemic α,α,β-trisubstituted β-lactones are the monomer units of poly((R,S)-3,3-dimethylmalic acid) (PDMMLA) derivatives, new biopolyesters showing great potential for biomedical applications. Using different groups during the synthesis of these β-lactones allows a tailored synthesis of PDMMLA copolymers with adjustable hydrophilic/phobic ratio. The degradation kinetics of the employed material is one of the most important criteria in the development of bioresorbable implants. The degradation time of PDMMLA derivatives can be controlled using different β-lactones of different hydrophilicity levels during the polymerization stage. Furthermore, PDMMLA has chemically available groups on its side chain allowing to graft functional groups on the polymer via covalent bonds. In this work, following a Steglich esterification protocol, the chemical grafting of cholesterol was carried out on a PDMMLA monomer derived β-lactone as well as on homopolymer PDMMLA–H, and copolymer PDMMLAH40-co-Hex60 (PDMMLA 40/60). Nuclear magnetic resonance (NMR) analyses of the products confirm and quantify the grafting ratio. 100% of cholesterol grafting has been realized on the homopolymer PDMMLA–H giving PDMMLA–Chol, and 10% on the copolymer PDMMLA 40/60, giving PDMMLAH30-ter-Chol10-ter-Hex60 (PDMMLA–Chol 30/10/60) as wished. Fourier-transform infrared (FT-IR) spectra, elemental analysis on the β-lactones and thermogravimetric analyses on the polymers also confirm the chemical modification of the products.


 Please wait while we load your content...
Please wait while we load your content...