Artificial sugar saccharin and its derivatives: role as a catalyst
Abstract
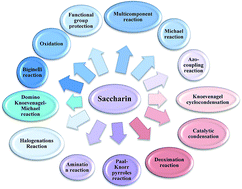
The primary objective of this review was to demonstrate the significance of artificial sugar saccharin and its derivatives as catalysts for a wide variety of organic transformations. The application of saccharin and its derivatives represents a greener and superior catalytic approach for reactions. In particular, we were interested in bringing together the literature pertaining to these saccharin derivatives from a catalysis perspective. The present review reports synthesis of saccharin and its derivatives such as saccharin-N-sulfonic acid, sodium saccharin, N-halo saccharin, saccharin lithium-bromide, N-formyl saccharin, N-acyl saccharin, N-nitrosaccharin, N-SCF3 saccharin, N-fluorosultam, N-phenylselenosaccharin, N-thiocyanatosaccharin palladium saccharin, DMAP–saccharin, and [Bmim]Sac. This catalytic application of saccharin and its derivatives includes reactions such as the Biginelli reaction, Paal–Knorr pyrrole synthesis, azo-coupling reaction, halogenations, domino Knoevenagel, Michael, deoximation reaction, catalytic condensation, functional group protection and oxidation etc. Also, these saccharin derivatives act as a source of CO, NH2, SCN, SCF3 and nitro groups. We reported all the available data on saccharin and its derivatives acting as a catalyst from 1957 to date.
- This article is part of the themed collection: 2020 Reviews in RSC Advances


 Please wait while we load your content...
Please wait while we load your content...