Electrochemically driven, cobalt–carbon bond-mediated direct intramolecular cyclic and acyclic perfluoroalkylation of (hetero)arenes using X(CF2)4X†
Abstract
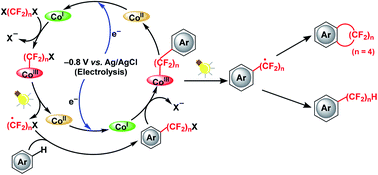
A proof-of-concept for the one-step, synthetically challenging cyclic and acyclic perfluoroalkylation of (hetero)arenes driven by the valence change of a vitamin B12 derivative as a cobalt catalyst in the presence of fluoroalkylating reagents (X(CF2)4X) is presented. The consecutive formation of cobalt–carbon bonds and generation of fluoroalkyl radicals by homolysis are the key steps for the reaction to proceed.


 Please wait while we load your content...
Please wait while we load your content...