A theoretical study of the hydrolysis mechanism of A-234; the suspected novichok agent in the Skripal attack†
Abstract
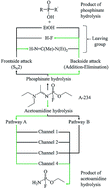
A-234, [EtO–P(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)(F)–N
O)(F)–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Me)–N(Et)2], is the suspected A-type nerve agent used in the Skripal attack on the 4th of March 2018. Studies related to the structure and reactivity of this compound are limited. We, therefore, aimed at understanding the underlying hydrolysis mechanism of A-234 within the DFT framework. The attack of the water molecule can occur at the phosphinate and acetoamidine reactive centres. Our theoretical findings indicate that the hydrolysis at the acetoamidine centre is thermodynamically favoured compared to the hydrolysis at the phosphinate centre. The hydrolysis at the acetoamidine moiety may proceed via two pathways, depending on the nitrogen atom participating in the hydrolysis. The main pathway consists of four distinct channels to reach the final product, with the concerted 1,3-proton shift favoured kinetically and thermodynamically in the gas phase and water as solvent. The results are in good agreement with the literature, although some differences in the reaction mechanism were observed.
C(Me)–N(Et)2], is the suspected A-type nerve agent used in the Skripal attack on the 4th of March 2018. Studies related to the structure and reactivity of this compound are limited. We, therefore, aimed at understanding the underlying hydrolysis mechanism of A-234 within the DFT framework. The attack of the water molecule can occur at the phosphinate and acetoamidine reactive centres. Our theoretical findings indicate that the hydrolysis at the acetoamidine centre is thermodynamically favoured compared to the hydrolysis at the phosphinate centre. The hydrolysis at the acetoamidine moiety may proceed via two pathways, depending on the nitrogen atom participating in the hydrolysis. The main pathway consists of four distinct channels to reach the final product, with the concerted 1,3-proton shift favoured kinetically and thermodynamically in the gas phase and water as solvent. The results are in good agreement with the literature, although some differences in the reaction mechanism were observed.


 Please wait while we load your content...
Please wait while we load your content...