Cathodic hydrogen production by simultaneous oxidation of methyl red and 2,4-dichlorophenoxyacetate aqueous solutions using Pb/PbO2, Ti/Sb-doped SnO2 and Si/BDD anodes. Part 1: electrochemical oxidation
Abstract
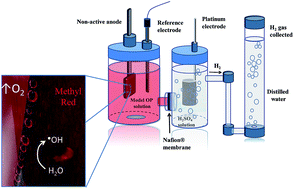
In this work, the electrochemical oxidation of the Methyl Red (MR) dye and the herbicide sodium 2,4-dichlorophenoxyacetate (2,4-DNa) was investigated on Si/BDD, Pb/PbO2 and Ti/Sb-doped SnO2 anodes in aqueous acidic medium by applying 30 mA cm−2 at 298 K. The electrochemical experiments were carried out in a two-compartment electrochemical cell separated through a Nafion® membrane (417 type) in order to use two types of supporting electrolyte to measure the elimination of the organic compound, the hydrogen production and the amount of oxygen produced during the oxidation of the pollutants. Although the main goal of this study is to understand the relationship between both processes, the evaluation of the current efficiencies (η) is a key parameter to determine the anodic oxidative capacity to degrade the proposed pollutants. The results clearly showed that MR and 2,4-DNa can be oxidized on Si/BDD, Pb/PbO2 and Ti/Sb-doped SnO2 anodes; however, significant variations in the oxidation level and η are achieved. Thus, although the MR solutions were completely discolored in all cases, only on the Si/BDD anode was MR oxidized to carboxylic acids in less than 15 min of electrolysis time. On Pb/PbO2 and Ti/Sb-doped SnO2 electrodes, the discoloration was slower and the oxidation was quasi-completed, leaving in solution some organic by-products, such as 2-aminobenzoic acid and/or N,N′-dimethyl-p-phenylenediamine, in the fixed electrolysis time. The behavior observed during the elimination of 2,4-DNa is due to its difficulty in degrading the chlorine groups in its aromatic ring which makes 2,4-DNa a more stable molecule. In the first oxidation stage, 2,4-dichlorophenol (2,4-DP) is produced in all cases, but on Si/BDD, this intermediate is quickly consumed. From the polarization curves and Tafel analysis, a reaction scheme for the formation and consumption of 2,4-DP was proposed.
- This article is part of the themed collection: Celebrating Latin American Talent in Chemistry


 Please wait while we load your content...
Please wait while we load your content...