Hybrid approach to obtain high-quality BaMO3 perovskite nanocrystals†
Abstract
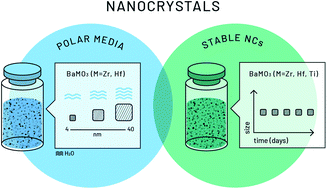
A novel hybrid solvothermal approach for perovskite nanocrystal formation via accurate control of the hydrolytic process is reported. This new synthetic methodology sets a whole general route to successfully tune the sizes of high-quality BaMO3 (M = Ti4+, Zr4+, and Hf4+) perovskite nanocrystals. Purely cubic-phase nanocrystals (stable in alcohol media) were obtained using controlled water amounts, combining the well-known aqueous sol–gel process with the classic solvothermal method. Exhaustive optimizations revealed feasibility of a fast (1 hour) and reproducible synthesis with small variations in the crystal size or agglomeration parameters. The study also reveals water content as the pivotal factor to achieve this wide range of sizes through a controlled hydrolytic step. Finally, the study of the hydrolytic process made it possible to shed some light on mechanistic insights of this synthetic route.


 Please wait while we load your content...
Please wait while we load your content...