Unexpected cyclization of ortho-nitrochalcones into 2-alkylideneindolin-3-ones†
Abstract
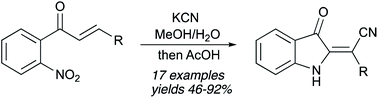
An original, facile, and highly efficient method for the preparation of 2-(3-oxoindolin-2-ylidene)acetonitriles from ortho-nitrochalcones is described. The featured transformation is a triggered Michael addition of the cyanide anion to the chalcone followed by a cascade cyclization mechanistically related to the Baeyer–Drewson reaction.


 Please wait while we load your content...
Please wait while we load your content...