Alkaline earth metal ion coordination increases the radical scavenging efficiency of kaempferol†
Abstract
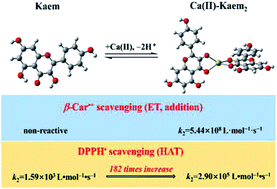
Flavonoids are used as natural additives and antioxidants in foods, and after coordination to metal ions, as drug candidates, depending on the flavonoid structure. The rate of radical scavenging of the ubiquitous plant flavonoid kaempferol (3,5,7,4′-tetrahydroxyflavone, Kaem) was found to be significantly enhanced by coordination of Mg(II), Ca(II), Sr(II), and Ba(II) ions, whereas the radical scavenging rate of apigenin (5,7,4′-trihydroxyflavone, Api) was almost unaffected by alkaline earth metal (AEM) ions, as studied for short-lived β-carotene radical cations (β-Car˙+) formed by laser flash photolysis in chloroform/ethanol (7 : 3) and for the semi-stable 2,2-diphenyl-1-picrylhydrazyl radical, DPPH˙, in ethanol at 25 °C. A 1 : 1 Mg(II)–Kaem complex was found to be in equilibrium with a 1 : 2 Mg(II)–Kaem2 complex, while for Ca(II), Sr(II) and Ba(II), only 1 : 2 AEM(II)–Kaem complexes were detected, where all complexes showed 3-hydroxyl and 4-carbonyl coordination and stability constants of higher than 109 L2 mol−2. The 1 : 2 Ca(II)–Kaem2 complex had the highest second order rate constant for both β-Car˙+ (5 × 108 L mol−1 s−1) and DPPH˙ radical (3 × 105 L mol−1 s−1) scavenging, which can be attributed to the optimal combination of the stronger electron withdrawing capability of the (n − 1)d orbital in the heavier AEM ions and their spatially asymmetrical structures in 1 : 2 AEM–Kaem complexes with metal ion coordination of the least steric hindrance of two perpendicular flavone backbones as ligands in the Ca(II) complex, as shown by density functional theory calculations.


 Please wait while we load your content...
Please wait while we load your content...