H2O-prompted CO2 capture on metal silicates in situ generated from SBA-15†
Abstract
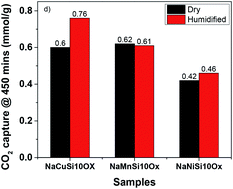
A series of metal silicates, NaMSi10Ox (M = Cu, Mn and Ni), were prepared by in situ doping of metals into mesoporous SBA-15 under a hydrothermal process, displaying a continuous framework of SiO4 structure with a narrow pore size distribution. These metal silicate materials were tested for CO2 adsorption behavior in the absence and presence of water. The results exhibited that the effect of H2O on the CO2 capture capability of metal silicates depends on the types of metal inserted into SBA-15. Compared to the dry condition, H2O addition enhances CO2 uptake dramatically for NaCuSi10Ox by 25%, and slightly for NaNiSi10Ox (∼10%), whereas little effect is shown on NaMnSi10Ox. The metal silicate materials are stable after adsorption of CO2 under wet conditions, which is benefited from their synthesis method, hydrothermal conditions. The improvement of CO2 uptake on metal silicates by H2O is attributed to the competitive and synergistic adsorption mechanism on the basis of IR investigations, where initially adsorbed H2O acts as a promoter for further CO2 capture through a hydration reaction, i.e., formation of bicarbonate and carbonates on the surface of the samples. These observations provide new possibilities for the design and synthesis of porous metal silicate materials for CO2 capture under practical conditions where moisture is present.


 Please wait while we load your content...
Please wait while we load your content...