Electronic properties and low lattice thermal conductivity (κl) of mono-layer (ML) MoS2: FP-LAPW incorporated with spin–orbit coupling (SOC)
Abstract
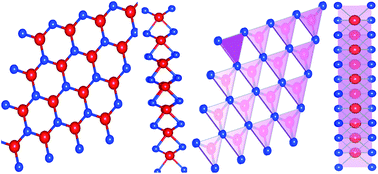
This paper focuses on the electronic and thermoelectric properties of monolayer MoS2. Here, we have examined the structure of MoS2, in which the hole in the center of the hexagonal cage is considered as a void atom, termed 1H-MoS2. Density functional theory (DFT) employing the generalized gradient approximation (GGA) and spin–orbit coupling (SOC) has been used for all calculations. Incorporation of SOC resulted in a significant change in the profile of the band energy, specifically the splitting of the valence band maximum (VBM) into two sub-bands. The “split-off” energy is found to be ∼20.6 meV. The reduction of the band gap with SOC is a prominent feature at the K–K location in the Brillouin zone. The band gap calculated with the GGA is ∼1.75 eV. However, on implementation of SOC, the GGA band gap was reduced to ∼1.68 eV. The frequency-dependent phonon dispersion curve was obtained to analyse the thermodynamical stability. 1H-MoS2 is found to be thermodynamically stable with no imaginary frequency. We report a low value of lattice thermal conductivity (κl) and low electron effective masses, which are desirable for potential applications in thermoelectric devices.


 Please wait while we load your content...
Please wait while we load your content...