High ionic conductivity of multivalent cation doped Li6PS5Cl solid electrolytes synthesized by mechanical milling†
Abstract
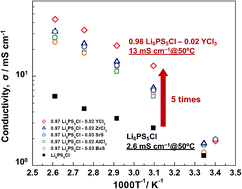
The performances of next generation all-solid-state batteries might be improved by using multi-valent cation doped Li6PS5Cl solid electrolytes. This study provided solid electrolytes at room temperature using planetary ball milling without heat treatment. Li6PS5Cl was doped with a variety of multivalent cations, where an electrolyte comprising 98% Li6PS5Cl with 2% YCl3 doping exhibited an ionic conductivity (13 mS cm−1) five times higher than pure Li6PS5Cl (2.6 mS cm−1) at 50 °C. However, this difference in ionic conductivity at room temperature was slight. No peak shifts were observed, including in the synchrotron XRD measurements, and the electron diffraction patterns of the nano-crystallites (ca. 10–30 nm) detected using TEM exhibited neither peak shifts nor new peaks. The doping element remained at the grain boundary, likely lowering the grain boundary resistance. These results are expected to offer insights for the development of other lithium-ion conductors for use in all-solid-state batteries.


 Please wait while we load your content...
Please wait while we load your content...