Two novel aromatic hydrocarbons: facile synthesis, photophysical properties and applications in deep-blue electroluminescence†
Abstract
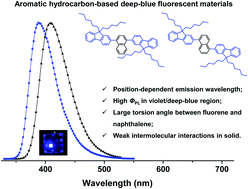
Two efficient novel fluorescent naphthalene and fluorene-based aromatic hydrocarbon isomers (1 and 2) are prepared and investigated for organic electroluminescence. These compounds show bright violet to deep-blue emission, narrow full width at half maximum (52 nm), and high photoluminescence efficiency (e.g. 0.61 in CH2Cl2, 0.67 in film). Alternation of substituent position on the naphthalene moiety can give rise to remarkable emission variation. The relatively large torsion angle between naphthalene and fluorene suppresses the π–π interactions by weakening the intermolecular interactions in the solid state, which can result in highly efficient fluorescence. Moreover, the 1931 Commission Internationale de L'Eclairage coordinates and maximum emission peak for deep-blue electroluminescence based on 1 are (0.16, 0.08) and 410 nm, respectively.


 Please wait while we load your content...
Please wait while we load your content...