Understanding the mechanism of the competitive adsorption in 8-methylquinoline hydrogenation over a Ru catalyst†
Abstract
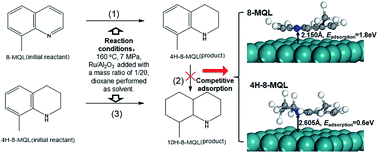
The competitive adsorption of 8-methylquinoline (8-MQL) and partially hydrogenated product, 4H-8-MQL, was studied by performing a combination of experiments and first-principles calculations over a selected Ru catalyst. A series of hydrogenation reactions were conducted with 8-MQL and 4H-8-MQL as initial reactants, respectively. 8-MQL exhibits stronger adsorption on catalyst surface active sites compared with 4H-8-MQL and the massive adsorption of 8-MQL hampers the further adsorption of 4H-8-MQL. The effects of temperature, pressure and solvent on the selectivity in 8-MQL hydrogenation were investigated as well. Full hydrogenation of 8-MQL to 10H-8-MQL was achieved within 120 min when the catalyst dosage increased from 5 wt% to 7 wt% under 160 °C and a hydrogen pressure of 7 MPa. The electronic charge of the N-heteroatom in 8-MQL and 4H-8-MQL was analyzed and the adsorption geometries of 8-MQL and 4H-8-MQL on the Ru(001) surface were optimized by DFT calculations to explain the competitive adsorption behaviors of 8-MQL and 4H-8-MQL.
- This article is part of the themed collection: 2020 RSC Advances HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...