Monte Carlo simulations using PELE to identify a protein–protein inhibitor binding site and pose
Abstract
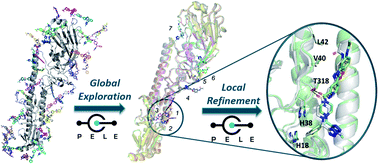
In silico binding site location and pose prediction for a molecule targeted at a large protein surface is a challenging task. We report a blind test with two peptidomimetic molecules that bind the flu virus hemagglutinin (HA) surface antigen, JNJ7918 and JNJ4796 (recently disclosed in van Dongen et al., Science, 2019, 363). Tests with a series of conventional approaches such as rigid (receptor) docking against available X-ray crystal structures or against an ensemble of structures generated by quick methodologies (NMA, homology modeling) gave mixed results, due to the shallowness and flexibility of the binding site and the sheer size of the target. However, tests with our Monte Carlo platform PELE in two protocols involving either exploration of the whole protein surface (global exploration), or the latter followed by refinement of best solutions (local exploration) yielded remarkably good results by locating the actual binding site and generating binding modes that recovered all native contacts found in the X-ray structures. Thus, the Monte Carlo scheme of PELE seems promising as a quick methodology to overcome the challenge of identifying entirely unknown binding sites and modes for protein–protein disruptors.


 Please wait while we load your content...
Please wait while we load your content...