Experimental and DFT studies of sulfadiazine ‘piano-stool’ Ru(ii) and Rh(iii) complexes†
Abstract
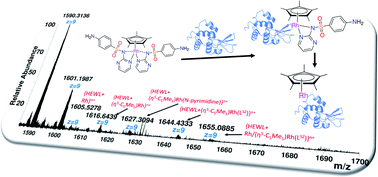
While sulfadiazine (HLSZ) is extensively used to elaborate complexes of intriguing biological applications (e.g. topical antibiotic silvadene; silver sulfadiazine), the molecular structure modification of sulfadiazine or even other sulfa drugs by coordination to either η6-cymene Ru(II) or η5-Cp* Rh(III) motif has not been investigated. Here, half-sandwich organoruthenium(II) and organorhodium(III) compounds of the type [(η6-p-cymene)Ru(LSZ)2] (1) and [(η5-C5Me5)Rh(LSZ)2] (2) are synthesized, characterized and evaluated for their potential antimicrobial activity. Spectroscopic and single crystal X-ray analysis showed that LSZ is coordinated to Rh(III) via both the sulfonamide and pyrimidine nitrogen atoms forming “piano-stool” geometry. In 2, the NMR equivalence clearly pointed to participation of two LSZ molecules in a fluxional process in which the third bond of the base of the stool is oscillating between two equivalent sulfonamide nitrogen atoms. While 1 was biologically inactive, complex 2 was potent against Gram-positive bacteria, Candida albicans and Cryptococcus neoformans. Hen white egg lysozyme (HEWL), a model protein, reacted covalently with 2 via the loss of one LSZ molecule, while compound 1 decomposed during the interaction with that protein.


 Please wait while we load your content...
Please wait while we load your content...