His18 promotes reactive oxidative stress production in copper-ion mediated human islet amyloid polypeptide aggregation†
Abstract
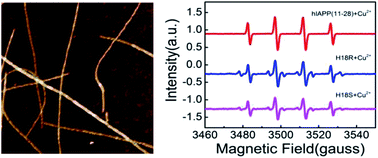
Copper ions play a critical role in human islet amyloid polypeptide (hIAPP) aggregation, which has been found in more than 90% of patients with type-2 diabetes (T2D). The role of Cu(II) in the cell cytotoxicity with hIAPP has been explored in two aspects: inhibiting the formation of fibrillar structures and stimulating the generation of reactive oxygen species (ROS). In this work, we carried out spectroscopic studies of Cu(II) interacting with several hIAPP fragments and their variants as well. Electron paramagnetic resonance (EPR) measurements and Amplex Red analysis showed that the amount of H2O2 generated in hIAPP(11-28) solution co-incubated with Cu(II) was remarkably more than hIAPP(1-11) and hIAPP(28-37). Furthermore, the H2O2 level was seriously reduced when His18 of hIAPP(11-28) was replaced by Arg(R) or Ser(S), indicating that His18 is the key residue of Cu(II) binding to hIAPP(11-28) to promote H2O2 generation. This is likely because the donation of electrons from the peptide to Cu(II) ions would result in the formation of the redox-active complexes, which could stimulate the formation of H2O2. Overall, this study provides further insight into the molecular mechanism of Cu(II) induced ROS generation.


 Please wait while we load your content...
Please wait while we load your content...