Sustained in vitro interferon-beta release and in vivo toxicity of PLGA and PEG-PLGA nanoparticles†
Abstract
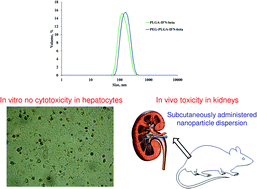
Interferon-beta-1a (IFN-β-1a) can diminish the symptoms of relapsing-remitting multiple sclerosis. Herein, we prepared sustained drug delivery IFN-β-1a-loaded nanoparticles by a double emulsion solvent evaporation method. Bovine serum albumin (BSA) model drug was used to optimize the preparation of nanoparticles composed of four types of poly(lactic-co-glycolic acid) (PLGA) polymers and two pegylated PLGA (PEG-PLGA) polymers. Via optimization, selected PLGA and PEG-PLGA polymers were able to entrap IFN-β-1a with high encapsulation efficiency (>95%) and low size (145 nm and 163 nm, respectively). In vitro release kinetics of BSA and IFN-β showed similar tendency for PLGA and PEG-PLGA nanoparticles, respectively. Although the drug loaded nanoparticles did not show toxicity in hepatocyte cells, mild toxic effects such as pale kidney and pyelectasis were observed in the in vivo studies.


 Please wait while we load your content...
Please wait while we load your content...