Ultrasonic-promoted enzymatic preparation, identification and multi-active studies of nature-identical phenolic acid glycerol derivatives†
Abstract
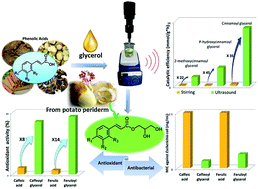
Phenolic acid glycerols (PAGs) are a group of rare phytochemicals found from potato periderm, which show great potential in the food, cosmetic and pharmaceutical industries. In this study, seven PAGs were enzymatically synthesized via transesterification of ethyl phenates (EPs) with glycerol by ultrasonic promotion. The conversions of 88.1–98.5% could be obtained in 1–9 h. Compared with the conventional stirring methods, the catalytic efficiency was significantly increased 11.0–44.0 folds by ultrasound assistance. The lipid peroxidation inhibition activity increased 8.1-fold and 14.4-fold compared to the parent phenolic acids (PAs). Furthermore, caffeoyl glycerol and feruloyl glycerol exhibited excellent antimicrobial activity against Escherichia coli compared to the corresponding PAs with minimum inhibitory concentration (MIC) decreasing 4–16-fold. The PAGs can also absorb a much wider and higher amount of the harmful UV-B rays than the corresponding PAs. The present strategy for facile synthesis of multifunctional PAGs paves the way for the development and application of natural phytochemicals and novel ingredients.


 Please wait while we load your content...
Please wait while we load your content...