Molecular flattening effect to enhance the conductivity of fused porphyrin tape thin films†
Abstract
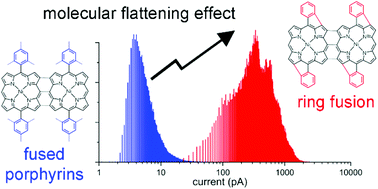
The straightforward synthesis of directly fused porphyrins (porphyrin tapes) from 5,15-diphenyl porphyrinato nickel(II) complexes with different substituents on the phenyl rings is achieved while processing from the gas phase. The porphyrin tapes, exhibiting NIR absorption, are readily obtained in thin film form. The gas phase approach cuts the need for solubilizing groups allowing for the first time the study of their conductivity according to the substituent. 2-Point probe and conductivity AFM measurements evidence that reducing the size of the meso substituents, phenyl < mesityl < di(3,5-tert-butyl)phenyl < di(2,6-dodecyloxy)phenyl, improves the thin film conductivity by several orders of magnitude. Density functional theory and gel permeation chromatography, correlate this improvement to changes in the intermolecular distances and molecular geometry. Furthermore, the oCVD of porphyrins with free ortho-phenyl positions causes intramolecular dehydrogenative side reactions inducing a complete planarization of the molecule. This molecular flattening drastically affects the π–π stacking between the porphyrins further enhancing the electronic properties of the films.


 Please wait while we load your content...
Please wait while we load your content...